Projects > Electrochemical Modification
The low-defect site density provided by our purification method facilitates novel electrochemical performance.¹ Electrochemical deposition of metal nanoparticles allows a great improvement in the interface between the SWNTs and metal contacts.
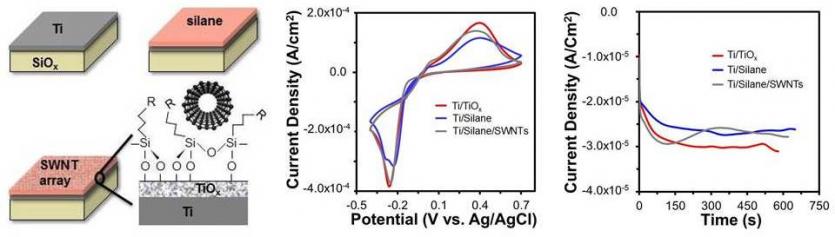
The interface was composed of a metal surface that had been modified with a self-assembled monolayer (SAM) which acted as an adhesion layer for the SWNTs (left). Cyclic voltammetry at this interface during various stages of modification revealed that while the silane alone reduced the electrochemical response relative to the bare surface, the SWNT network served to improve the electrochemical sensitivity during metal nanoparticle deposition (middle). The information obtained from cyclic voltammetry was used to choose appropriate deposition potentials for copper nanoparticle formation (right). The sigmoidal shape for the current vs time traces for the SWNT-modified samples, indicated enhanced instantaneous and progressive nucleation processes
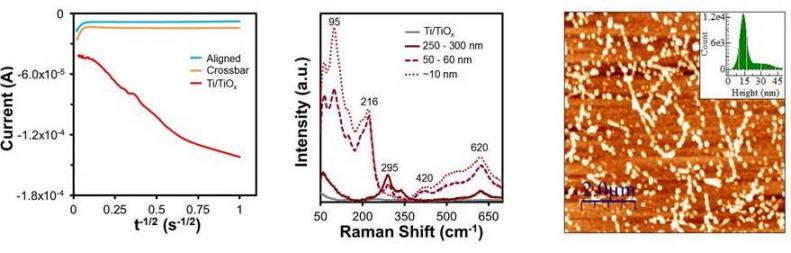
Cottrell plots (i vs √t) of the current response recorded during nanoparticle growth showed that when the charge allowed to pass was limited to 16.7 millicoulombs (mC), Ti/TiOx samples behaved as macroscopic electrodes, while the electrochemically isolated SWNT networks behaved as an array of nanoelectrodes (left). Raman microscopy (middle) revealed that the dominate oxidation state of the metal nanoparticles varied with size. AFM images of a SWNT-modified sample showed that the deposition of a low-density network of SWNTs provided nucleation points for metal nanoparticles along the conductive sidewalls of the nanotubes, as observed from the greatly increased density of nanoparticles observe (right).
1. D. Asheghali, P. Vichchulada, and M. D. Lay, J. Am. Chem. Soc., 135, (2013), 7511

