Aerosolized nanoparticles
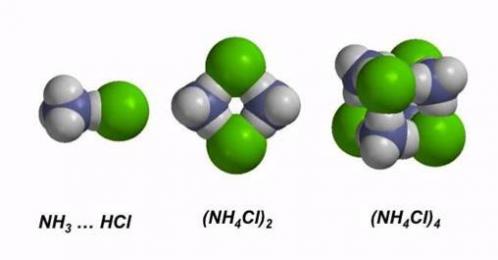 Nanoparticles of ammonium chlorides, nitrates, sulfates and other salts are formed in the atmosphere as a result of acid-base reactions between reagents from natural and human sources. Our current research focuses on their structures and reactivity trends as a function of size, concomitantly revealing new information about intermolecular forces. Our latest work (see (1) and (2) below) indicates that ammonium fluoride nanoclusters exhibit three different kinds of hydrogen bonds, depending on the size and charge state of the particle.
Nanoparticles of ammonium chlorides, nitrates, sulfates and other salts are formed in the atmosphere as a result of acid-base reactions between reagents from natural and human sources. Our current research focuses on their structures and reactivity trends as a function of size, concomitantly revealing new information about intermolecular forces. Our latest work (see (1) and (2) below) indicates that ammonium fluoride nanoclusters exhibit three different kinds of hydrogen bonds, depending on the size and charge state of the particle.
We have just released TransRot, machine-portable software for carrying out simulated annealing Monte Carlo optimizations of these systems. Please visit https://github.com/steventopper/Transrot .
(1) A.J.V. Lomboy* and R.Q. Topper, Nonuniform proton transfer and strong hydrogen bonding within cation, anion, and neutral clusters of ammonia and hydrogen fluoride, Journal of Physical Chemistry A, 125 (12), 2546-2557 (2021).
(2) J.J. Biswakarma*, V. Ciocoi* and R.Q. Topper, Energetics, thermodynamics, and hydrogen bond diversity in ammonium halide clusters, 120(40), pp. 7924-7934 (2016).
(3) R.Q. Topper, W. V. Feldmann*, I. Markus*, D. Bergin*, and P.R. Sweeney*, Simulated annealing and density functional theory calculations of structural and energetic properties of the ammonium chloride clusters (NH4Cl)n, (NH4+)(NH4Cl)n and (Cl–)(NH4Cl)n, n = 1–13, Journal of Physical Chemistry A, 115 (38), pp. 10423-10432 (2011).
(4) R.Q. Topper, D.L. Freeman, D. Bergin* and K. LaMarche**, Computational techniques and strategies for Monte Carlo thermodynamic calculations with applications to nanoclusters, invited book chapter, Reviews in Computational Chemistry, Vol. 19, pp. 1-41, K.B. Lipkowitz, R. Larter and T.R. Cundari, Eds., Wiley-VCH/John Wiley and Sons, New York (2003). ISBN 0-471-23585-7.
(5) A. Matro, D.L. Freeman, and R.Q. Topper, Computational study of the structures and thermodynamic properties of ammonium chloride clusters using a parallel J-walking approach,Journal of Chemical Physics104, 8690 (1996).
* = Undergraduate student researcher at the time the research was initiated.
Last edit 4/1/21

